Chelate?
The first time I came across this word "chelate" was when I was busy gathering various information related to the organic method to strengthen the soil right after I finally set up a small vegetable bed in a corner of my garden and became a proud farmer. Then, I encountered this term again in the articles regarding the rate of absorption in the body of
some trace mineral supplements. I knew I could properly understand this concept only with serious brush up on chemistry basics, so I just put it aside, but for some reason, I had an ominous feeling that I might have to come back to it soon in the near future.I have been successfully sprouting mung beans in a super easy way with literally no hassle every time without a fail, using a simple yet very clever method that I came to know by chance a long time ago. I've been meaning to write a post about this to share with other people especially since I saw the price of a pack of sprout at local market. And with that, I thought it'd better explain the awesome benefits of germination. However, it would be also fair to mention the dark side of mung bean being tagged as anti-nutrient. Then, the word "chelate" pops up again. OK.. it's the third time...and 3 times is a fate. I finally decided to go down the rabbit hole of "chelate". Brushing up on chemistry basic feels like rendezvous with your ex boyfriend... best to avoid if you can...
“Chelate”
One of the categories to classify the way of bonding in chemistry is covalent bonding1) and coordinate covalent bonding. While in covalent bonding, each side atom provide an electron and share a pair of electrons which is attracted by both of the nuclei, in coordinate covalent bonding, only one side atom offer a pair of electrons(called Lewis base lone pair) to the other atom(called Lewis acid). In water molecule(H2O), we can see both of the bonding. Covalent bond involves non metallic atoms and coordinate covalent bond involves metallic atoms.
All organic matters in this world are made up by combining heteroatoms with Carbon and Hydrogen. Oxygen(O), Nitrogen(N), Sulfur(S), Phosphorus(P), Halogen(Cl, F, and etc.), are called Hetero atoms. Unlike Carbon and Hydrogen, they perform coordinate covalent bonding(2 lone pairs of electrons in Oxygen in the above image). Hetero atoms offer a pair of electrons(electron donor) to a metal cation(electron acceptor). This metal cation lacks electrons and eagers to fill the missing orbitals to become stable. Imagine a metal atom which usually wanders around in a positive ion state(cation) after losing electrons meets a donor atom(ligand). One ready to give, the other ready to accept. Perfect! They bond. The part bonded to the central metal with its lone pair of electrons is called a ligand and if there are multiple ligands, that is to say if the bonding is performed in various places, the bonding must be tighter and stronger. When this bonding happens at more than 2 ligands, we call it "chelate". The more ligands, the stronger bonding. The most widely and commonly used chelate EDTA (Ethylene Diamine Tetra Acetic Acid) has 4 ligands.
Basically chelate describes a complex(coordination compound) formed with a metal or metal cation in the center and its surrounding ligands. So chelate is about metal, namely mineral. In our daily life, chelate is utilized to deal with various situation involving metals or minerals using that specific bonding ability. I went to further to see how they are used in real life. Surprisingly, they are so close to us...
1. Agriculture: Improving access to nutrients for crops
Phosphorous can precipitate with minerals such as calcium, copper, zinc, and manganese in the soil and end up creating an insoluble form, in which state crops cannot actually utilize them easily. Therefore, during the process of making fertilizer by adding chelating ingredients to chelate these mineral cations to prevent them from attaching to phosphoric acid, plants can better absorb these minerals.2) Usually, organic acids such as citric acid, fluvic acid, ammonia, and EDTA are used as a chelating agent.

Additionally, where excessive use of fertilizers causes serious salt accumulation in the soil and as a result nutrients from the newly applied fertilizer becomes insoluble, crops cannot absorb them from the root. Also in this case, chelates can be used to transform metals, or minerals, into a water-soluble form so that plants can actually absorb. There are various products on the market based on this principle.
2. Preventing oxidation(rancidity) of cooking oil
It is said that there exists some, although very little, trace amounts of metal ions in cooking oil, which can act as a catalyst to make the oil oxidated3). So the addition of a chelating agent such as EDTA can prevent it from acting as a catalyst by locking the metal ion.
3. Release of harmful heavy metals from the body
Harmful heavy metals(lead, mercury, arsenic and cadmium) and POPs(Persistent Organic Pollutants) have become part of our lives even if we try our best to avoid them. Currently, many hospitals, especially functional medicine practitioners, are offering procedures to detect and measure heavy metals in our bodies to eliminate and detoxify them. Chelation therapy is carried out by injecting chelating substances intramuscularly or intravenously to bind the target heavy metal to expel out of the body. Depending on the type of heavy metal to work on, various chelating agents are used :Dimercaprol (British Anti-Lewisite, BAL),
DMSA(dimercaptosuccinic acid or succimer) and DMPS (dimercaptopropane sulfonate), Penicillamine and EDTA.4)
4. Medical: blood anticoagulant
"Anticoagulants are used to prevent clot formation both in vitro and in vivo. Calcium is necessary for a wide range of enzyme reactions of the coagulation cascade and its removal irreversibly prevents blood clotting within the collection tube".5) EDTA prevents coagulation by removing or chelating calcium from the blood. EDTA is recommended as the most suitable coagulation for blood test since it preserves cellular components of blood cells.
5. Home: Improved water quality for efficient washing
The water we use is classified into hard water and soft water depending on the level of metals, mainly calcium (Ca2+ ) and magnesium (Mg2+ ). When these two metals, which are abundant in hard water, come into contact with soap or detergent, they turn into calcium salts and magnesium salts, making it difficult to foam, and as a result more detergents are required, which is undesirable. It is said that it can even damage your clothes. In this case, a chelating agent such as EDTA, which binds very well to metals, is added when making detergents to trap calcium or magnesium so that they no longer have ionic properties. The calcium is then deactivated and the water becomes like soft water, allowing more efficient washing.6) The same goes for soap.
6. Enhancing bleaching efficiency in chemical pulp for paper
Most of the chemical pulps used today for paper, fiber, or the production of cellulose derivatives need to undergo a bleaching process in order to achieve desired brightness and for that oxygen-based bleaching substances(ozone, hydrogen peroxide, oxygen, peracids) are used. However, the transition metal present in the pulp such as manganese (Mn2+) , copper (Cu2+) , and iron (Fe2+) react with them and cause oxidation resulting lowering quality of paper and can catalytically degrade the breaching agents resulting low efficiency in breaching7). So, they must be removed or at least neutralizes before the actual bleaching to prevent the undesirable oxidation and degradation of cellulose. For that chelating agent like EDTA or DTPA(diethylenetriaminepentaacetic acid) is used.
7. Increasing the absorption rate of nutrients
What if we don't have enough amount mineral in our body? From making bones and muscles to regulating optimal function of immune system and inflammation, it's like a Jack of all trades in the living organism. And even more importantly, what if we do take enough amount minerals but somehow they fail to enter the membranes of the small intestines to be utilized? This has been unfortunately the case for most of us.
How can we herd excited mineral ion straight to the intestines without giving them any opportunity to bind whatever compound they encounter? By chelating them with the carrier. What condition needs to be met to be the most reliable chelating agent in this case? First of all, our body is consists of 70% of water and the they should be in "soluble" state. Secondly, to penetrate into the intestines wall through integral protein, only mineral carrier with the molecular weight smaller than that of integral protein can penetrate the cell membrane and permeate from the outside to the inside. Satisfying these conditions, Oligopeptides with less than 9 amino acid molecules is soluble in water, so can be eligible carrier of mineral all the way to the inside of cells. Among the amino acid, Glycine is the smallest amino acid and maybe that explain the varieties of calcium glycinate, magnesium glycinate, zinc glycinate, ferrous glycinate, iron glycinate, copper glycinate and manganese glycinate. The image below is information about chelated magnesium supplements I'm taking.

In a study designed to determine whether replacing inorganic trace minerals with supplements based on chelation technology in chickens affects their growth, mineral digestibility, bone quality and antioxidant status, it is reported that chelated minerals had a significantly positive benefits.8) I think this is a meaningful results considering that the key in taking nutritional supplements is how much they are actually absorbed and utilized by our cells. Wouldn't it be correct to say "I am what my body actually absorbs" rather than "I am what I eat"?
Chelates are deeply embedded in our lives in various ways, whether we aware of it or not, such as in cosmetics, preservatives, and contrast agents for MRI scans9) just to name a few. As always, everything that makes our lives more convenient and enriched comes with a inevitable price tag. It has always been that way, and will continue to be. While looking into chelates, I saw many studies pointing out that the possible side effects and dangers that we should address and deal with before carrying on using chelates. In particular, issues related to environmental issues caught my attention. This is an area I want to learn more about little by little. Green chelate may be the way to go forward.
Now, let's go back to the main topic I originally wanted to mention...
Seeds have defense system.
In nature, there is nothing that exists for no reason. Plants, unlike animals under the constraint of being unable to leave and move around from one place for the rest of their lives, must have evolved its own way to ensure geminate and reproduce offspring to pass their genes to future generation. They might have needed devices like a digestive enzyme inhibitor so their seeds can survive inside the digestive tract and come out intact with faeces when eaten by predator animals. They might have found the way to make predators to spit the seeds out by putting some terrible taste or odor or even make them have diarrhea by adding some toxics. Loads of ingenious devices to keep the predators in check including poisons if necessary as their own protective measures.
In addition, in order to successfully germinate and grow well, they would have wanted to store sufficient nutrients inside the seeds in advance. A friend of mine once said how awesome all the energy to become a big plant is compressed in such a small seed. It is in deed. The explosive energy contained in that small seed is truly amazing. Plants would have also made sure these precious seeds can be protected from possible molds or bacteria in the soil by adding some antioxidant substances.
It makes perfect senses and absolutely understandable if I put myself into the shoes of plants. It's just that I, as a human, am a predator to them. I'm at the other side of the playground.
Phytate(or phytic acid)
Well, brown rice, unprocessed grains (kamut, spelt, oats, etc.), sesame seeds, pumpkin seeds, flax seeds, hemp seeds, chia seeds, Brazil nuts, walnuts.... make up a large portion of our family's staple food. Not a good news. You might heard someone saying integral rice is poison. That sounds a bit of exaggeration. Instead of conducting witch hunting, further investigation seems more reasonable.
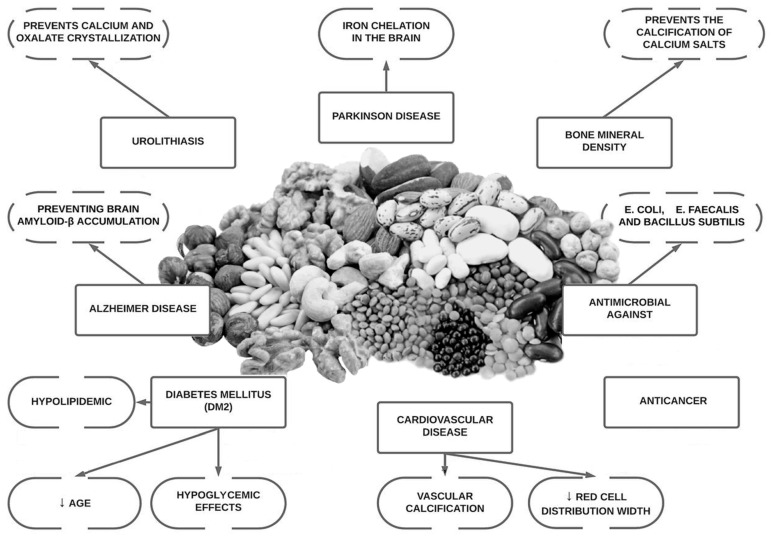
First, to keep things fair and balanced, let’s also look into the benefits of phytic acid. Fortunately, there are plenty of research results published in favor of them. In addition to the antioxidant10), immune system strengthening, and anti-cancer effect11), there are also research results suggesting the consumption of foods rich in phytic acid can help lower the risk of urinary stones by inhibiting the crystallization of calcium salts.12) In various articles, it is suggested phytic acid can inhibit vascular calcification, urolithiasis, osteoporosis, and play positive influence over cognitive function, neurodegenerative diseases, cancer tumor formation, type 2 diabetes, and overall cardiovascular health.13) It certainly helps my making a choice to keep seeds and nuts close by.
In addition to the benefits that phytic acid can bring, the aspect of interference with mineral absorption is also being studied. Many studies have detected presence of Bifidobacteria in the human intestine that can decompose phytic acid or further degrade six phosphates (IP6) to produce phosphate intermediates (IP(3) ~IP(5)).14), 15) No doubt it is a pleasing information since by maintaining a healthy and balanced gut environment, we can count on the activity of phytase, an enzyme that decomposes phytic acid.
I've come to learn a lot so far. But lastly, I wondered if there is something I can do at home to ease my concern over those food with phytic acid? I also found many articles on this topics as well. To put them very briefly,
1. Let’s soak them in warm water. It is even better to add an acid such as vinegar or citric acid.
2. Let’s germinate.
3. Let’s ferment.
The article got too long. The rabbit hole of chelation and phytic acid was quite deep and long and I got lost!
In the next post, I will talk about sprouting, which was the reason I started this article in the first place.
Ah... Who would have thought the story about sprouting bean would be such a long story...
References
2) Chelation in Agriculture Fertilizers: What to know
https://andersonsplantnutrient.com/agriculture/market-feed/4911
3) Chelating agents of Food https://www.biosciencenotes.com/chelating-agents-of-food/
4) Chelation: Harnessing and Enhancing Heavy Metal Detoxification—A Review
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3654245/
5) The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes
https://pubmed.ncbi.nlm.nih.gov/17484616/
6)Safe House Cleaning: What is Chelation?
https://www.safehouseholdcleaning.com/what-are-chelating-agents/
7) Evaluating chelating agents and their effects on cellulosic pulps during P-stage bleaching.
https://link.springer.com/article/10.1007/s10570-023-05110-1
8) Effect of advanced chelate technology based trace minerals on growth performance, mineral digestibility, tibia characteristics, and antioxidant status in broiler chickens
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7596981/
9) Current Clinical Issues: Deposition of Gadolinium Chelates
https://www.intechopen.com/chapters/71099
https://www.sciencedirect.com/science/article/abs/pii/089158499090146A?via%3Dihub
11) Protection against cancer by dietary IP6 and inosito
https://pubmed.ncbi.nlm.nih.gov/17044765/
12)Renal lithiasis and nutrition
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1586208/
13) Phytate Intake, Health and Disease:“Let Thy Food Be Thy Medicine and Medicine Be Thy Food”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9855079/
14) Myo-inositol hexakisphosphate degradation by Bifidobacterium infantis ATCC 15697
https://pubmed.ncbi.nlm.nih.gov/17462768/
15) Phytate degradation by human gut isolated Bifidobacterium pseudocatenulatum ATCC27919 and its probiotic potential
https://www.sciencedirect.com/science/article/abs/pii/S0168160509003614?via%3Dihub